New possibilities for hydrogen-producing algaeMar 25, 2009 - Science Daily
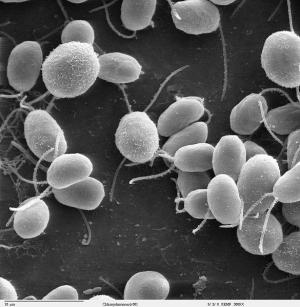
Photosynthesis produces the food that we eat and the oxygen that we breathe ― could it also help satisfy our future energy needs by producing clean-burning hydrogen? Researchers studying a hydrogen-producing, single-celled green alga, Chlamydomonas reinhardtii, have unmasked a previously unknown fermentation pathway that may open up possibilities for increasing hydrogen production. In the new study by Dubini et al., published in the Journal of Biological Chemistry, researchers at the Carnegie Institution's Department of Plant Biology, the National Renewable Energy Laboratory (NREL), and the Colorado School of Mines (CSM), examined metabolic processes in a mutant strain that was unable to assemble an active hydrogenase enzyme. The researchers, who include Alexandra Dubini (NREL), Florence Mus (Carnegie), Michael Seibert (NREL), Matthew Posewitz (CSM), and Arthur Grossman (Carnegie), expected the cell's metabolism to compensate by increasing metabolite flow along other known fermentation pathways, such as those producing formate and ethanol as end products. Instead, the algae activated a pathway leading to the production of succinate, which was previously not associated with fermentation metabolism in C. reinhardtii. Notably, succinate, a widely used industrial chemical normally synthesized from petroleum, is included in the Department of Energy's list of the top 12 value added chemicals from biomass. "We actually didn't know that this particular pathway for fermentation metabolism existed in the alga until we generated the mutant," says Carnegie's Arthur Grossman. "This finding suggests that there is significant flexibility in the ways that soil-dwelling green algae can metabolize carbon under anaerobic conditions. By blocking and modifying some of these metabolic pathways, we may be able to augment the donation of electrons to hydrogenase under anaerobic conditions and produce elevated levels of hydrogen." Grossman points out that it makes evolutionary sense that a soil organism such as Chlamydomonas would have a variety of metabolic pathways at its disposal. Oxygen levels, nutrient availability, and levels of metals and toxins can be extremely variable in soils, over both the short and long term. "In such an environment", Grossman says, "these organisms must evolve flexible metabolic circuits; the variety of conditions to which the organisms are exposed might favor one pathway for energy metabolism over another, which would help the organism compete in the soil environment over evolutionary time." Grossman led the effort to generate a fully sequenced Chlamydomonas genome, which has allowed researchers to identify key genes encoding proteins involved in both fermentation and hydrogen production. Grossman feels that it is of immediate importance to generate new mutant strains to help us understand how we may be able to alter fermentation metabolism and the production of hydrogen. NREL's Michael Seibert, the project's Principal Investigator, observed that "the overarching goal of the work is to gain a fundamental understanding of the total suite of metabolic processes occurring in Chlamydomonas and how they interact; this discovery effort will lead to the development of novel ways to produce renewable hydrogen and other biofuels, which will benefit all of us". "These are really exciting times in the field," says Matthew Posewitz. "The tools developed at Carnegie and by other groups in the field are presenting unprecedented opportunities for scientists to make important advances in our understanding of the basic biology of organisms such as Chlamydomonas." As an energy source to potentially replace fossil fuels, hydrogen would greatly reduce the emission of greenhouse gases. Proponents of algal-based hydrogen production point out that, unlike ethanol produced from crops, it would not compete with food production for agricultural land. The Project is being supported by the US Department of Energy's GTL Program within the Office of Biological and Environmental Research. Journal reference:
Adapted from materials provided by Carnegie Institution, via EurekAlert!, a service of AAAS.
|
Email this page to a friend
If you speak another language fluently and you liked this page, make
a contribution by translating
it! For additional translations check out FreeTranslation.com
(Voor vertaling van Engels tot Nederlands)
(For oversettelse fra Engelsk til Norsk)
(Для дополнительных
переводов проверяют
FreeTranslation.com )